

In octahedral symmetry the d-orbitals split into two sets with an energy difference, Δ oct (the crystal-field splitting parameter, also commonly denoted by 10 Dq for ten times the "differential of quanta" ) where the d xy, d xz and d yz orbitals will be lower in energy than the d z 2 and d x 2- y 2, which will have higher energy, because the former group is farther from the ligands than the latter and therefore experiences less repulsion. The most common type of complex is octahedral, in which six ligands form the vertices of an octahedron around the metal ion. The stronger the effect of the ligands then the greater the difference between the high and low energy d groups. the nature of the ligands surrounding the metal ion.the coordination number of the metal (i.e.

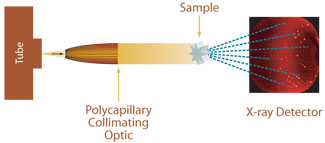
the arrangement of the ligands around the metal ion.A higher oxidation state leads to a larger splitting relative to the spherical field. This splitting is affected by the following factors: Thus the d-electrons closer to the ligands will have a higher energy than those further away which results in the d-orbitals splitting in energy. The electrons in the d-orbitals and those in the ligand repel each other due to repulsion between like charges. As a ligand approaches the metal ion, the electrons from the ligand will be closer to some of the d-orbitals and farther away from others, causing a loss of degeneracy. The theory is developed by considering energy changes of the five degenerate d-orbitals upon being surrounded by an array of point charges consisting of the ligands. CFT can be complicated further by breaking assumptions made of relative metal and ligand orbital energies, requiring the use of inverted ligand field theory (ILFT) to better describe bonding.Īccording to crystal field theory, the interaction between a transition metal and ligands arises from the attraction between the positively charged metal cation and the negative charge on the non-bonding electrons of the ligand. CFT was subsequently combined with molecular orbital theory to form the more realistic and complex ligand field theory (LFT), which delivers insight into the process of chemical bonding in transition metal complexes. CFT was developed by physicists Hans Bethe and John Hasbrouck van Vleck in the 1930s. CFT successfully accounts for some magnetic properties, colors, hydration enthalpies, and spinel structures of transition metal complexes, but it does not attempt to describe bonding. This theory has been used to describe various spectroscopies of transition metal coordination complexes, in particular optical spectra (colors). We request partial support for a PDRA to make the best use of the equipment, to which the University and the School will contribute a total of 50,000.In molecular physics, crystal field theory ( CFT) describes the breaking of degeneracies of electron orbital states, usually d or f orbitals, due to a static electric field produced by a surrounding charge distribution (anion neighbors). The present proposal is an underpinning technology in all these areas. The new School of Pharmacy, for which staff recruitment has already started, is to be housed in an extension to the chemistry building, and is expected to generate new programmes in medicinal chemistry and drug delivery. Within medicinal and biological chemistry, funded programmes include the development of bisintercalating antitumour agents, novel DNA structural motifs and carbohydrate chemistry. Within materials, there are specific funded research programmes in high performance polymers, metal cyanides, open framework metal phosphates and analogues, organometallic molecular wires, selective complexation of actinides and nuclear fission products. The two broad areas which will benefit most within the School are in materials and in medicinal and biological chemistry. We seek to support a range of currently funded projects for which our existing 10 year old image plate equipment is inadequate, and also to develop new projects. W e seek funding for a single crystal diffractometer with CCD detector to provide a major enhancement of our structure determination capability for small molecules. Single-crystal X-ray Diffraction for the Chemical Science


 0 kommentar(er)
0 kommentar(er)
